- 骨髓增生异常综合征(MDS)怎么治疗?MDS能治好吗?
- 发布于 2015-02-04 11:29 来源:刘新建医生
我们如何治疗高危骨髓增生异常综合征(MDS)?作者:迈克尔 A.Sekeres 克利夫兰临床肿瘤研究所(读书心得)河南省肿瘤医院血液科刘新建
2014年12月6日在美国旧金山参加美国血液病年会期间,有幸聆听了美国克利夫兰临床肿瘤研究所的迈克尔教授的《骨髓增生异常综合征的治疗及新药进展》,感觉很受鼓舞。今天再次找出迈克尔教授发表在2014年2月的《BLOOD》上的《我们如何治疗高危骨髓增生异常综合征》拜读,现将读书心得给大家共享:
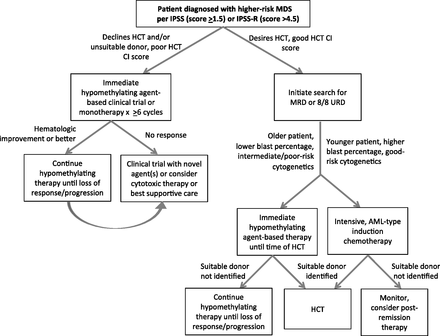
高危骨髓增生异常综合征(MDS)是指按照IPSS评分:中危-2、高危组的MDS患者。或者IPSS-R评分:高危、非常高危及部分中危患者。或者按照WHO组织学分类RAEB-1RAEB-2患者。这组患者生存时间较短,一旦确诊,应尽快进行治疗。这组患者的标准治疗方案是去甲基化治疗,有合适供者及身体条件合适者应着手进行造血干细胞移植。去甲基化治疗的药物主要有阿扎胞苷、地西他滨。这两种药物至少要应用6个周期,然后继续每三个月进行一次维持治疗,直到患者对药物无反应。患者如果对药物无反应,其他的治疗方法有限,患者的生存期也很短。这时应该考虑临床试验,进行新的药物治疗。
患者如果体能状态很好,无明显的共患病,有合适的造血干细胞供者,应该提前进行造血干细胞移植准备。至于移植前治疗,去甲基化治疗及诱导化疗均要进行,或者进入临床试验。尽管移植前的最好治疗方法我们换不知道,但是这些治疗可以阻止疾病进展。
How we treat higher-risk myelodysplastic syndromes
Mikkael A. Sekeres and Corey Cutler
Higher-risk myelodysplastic syndromes (MDS) are defined by patients who fall into higher-risk group categories in the original or revised International Prognostic Scoring System. Survival for these patients is dismal, and treatment should be initiated rapidly. Standard therapies include the hypomethylating agents azacitidine and decitabine, which should be administered for a minimum of 6 cycles, and continued for as long as a patient is responding. Once a drug fails in one of these patients, further treatment options are limited, median survival is<6 months, and consideration should be given to clinical trials. Higher-risk eligible patients should be offered consultation to discuss hematopoietic stem cell transplantation close to the time of diagnosis, depending on patient goals of therapy, with consideration given to proceeding to transplantation soon after an optimal donor is located. In the interim period before transplantation, hypomethylating agent therapy, induction chemotherapy, or enrollment in a clinical trial should be considered to prevent disease progression, although the optimal pretransplantation therapy is unknown.
Introduction
The myelodysplastic syndromes (MDS) are the most commonly diagnosed myeloid neoplasms in the United States, with an incidence rate of 4.6 in100 000 US citizens, translating to approximately 15 000 new diagnoses yearly.1 This figure is often considered to be an underestimate, because data derived from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program and the North American Association of Central Cancer Registries are likely compromised by under-reporting (thought to be a result of misconceptions about the disease’s neoplastic basis and variability in diagnostic prowess) and misclassification (as evidenced by the 50% of patients in such registries identified as “MDSCunclassifiable”).2,3
MDS represents a constellation of diagnoses increasingly identified by underlying genetic abnormalities, such as the del(5q) syndrome, SF3B1 mutations in MDS with ring sideroblasts along with other splicing factors, abnormalities along tyrosine kinase pathways (such as CBL and NRAS), mutated genes involved with epigenetic dysregulation (TET2, DNMT3A, EZH2, IDH1 and 2, and ASXL1), and mutations in transcription factors (RUNX1, ETV6)4⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-33; and on a disease biology that at some extremes is typified by excessive production of proapoptotic, proinflammatory cytokines and premature death of hematopoietic stem cells, and at others by excessive proliferations, epigenetic regulation, and a block in differentiation.34⇓⇓⇓-38 Molecular data are becoming disease defining (as with spliceosome mutations in MDS with ring sideroblasts), have been incorporated into prognostic scoring systems and are anticipated to provide additional resolution to these systems, and have been linked to therapeutic responsiveness (discussed later). However, the degree to which they will modify risk estimates in MDS is being explored by an international working group.5,7,22,39,40
Treatment decisions in MDS are based on pathology, or a prognostic scoring system appropriated as a default staging system, and are now incorporated into drug labeling.41 As a result, the classification of MDS patients has become reductionist, with patients divided into those with lower-risk or higher-risk disease, as determined by prognostic systems that are based most commonly on blast percentage, cytogenetic risk groups, and cytopenias, but which may also include age, performance status, transfusion needs, and other clinical (and increasingly molecular) factors.42⇓-44 Patients with higher-risk disease fall into International Prognostic Scoring System (IPSS) categories of Intermediate-2 and High groups, corresponding largely to IPSS-R groups Very High, High, and, sometimes, Intermediate, and which often correspond to World Health Organization (WHO) histologic subtypes of refractory anemia with excess blasts (RAEB)-1 and RAEB-2, with an expected median overall survival of<2 years.4,41,45 Whether survival estimates can be adjusted within the modern therapeutic era has not yet been determined. Correlations between IPSS/IPSS-R and WHO classifications are loose, because some patients with excess blasts but normal karyotype and limited cytopenias can live for years, whereas those with few blasts, complex karyotype, and profound cytopenias may have a shortened survival rate.
Treatment options for patients with lower-risk MDS have recently been reviewed.46 The treatment of an MDS patient with higher-risk disease starts with recognition of the imperative to initiate therapy. Accepting the premise that the IPSS is a default MDS staging system, with Low-High reflecting stages I-IV, and comparing it stage-for-stage with American Joint Committee on Cancer staging for nonCsmall-cell lung cancer, overall survival is worse for patients with MDS.47,48 Just as it would be poor practice in a patient with stage III or IV lung cancer, acceptable comorbidities, a good performance status, and a desire to receive treatment to recommend watchful waiting simply because that patient does not yet have debilitating symptoms, so too would therapy avoidance be discouraged in a similar MDS patient with Intermediate-2 or High risk of disease. We offer examples of 2 patients and answer the typical questions posed to us by informed patients to illustrate how we approach higher-risk MDS.